Resources
Events
- Orange County Convention Center, Orlando, FloridaDecember 6–9, 2025American Society of HematologyASH Annual Meeting and Exposition
 Tampa Marriott AirportFebruary 27–March 1, 2026CHES FoundationOne Drop Rare Bleeding Disorders Consortium
Tampa Marriott AirportFebruary 27–March 1, 2026CHES FoundationOne Drop Rare Bleeding Disorders Consortium Oregon Convention Center, Portland, Oregon & OnlineMarch 19-21, 2026THSNA 2026Thrombosis & Hemostasis Summit of North America
Oregon Convention Center, Portland, Oregon & OnlineMarch 19-21, 2026THSNA 2026Thrombosis & Hemostasis Summit of North America
Publications


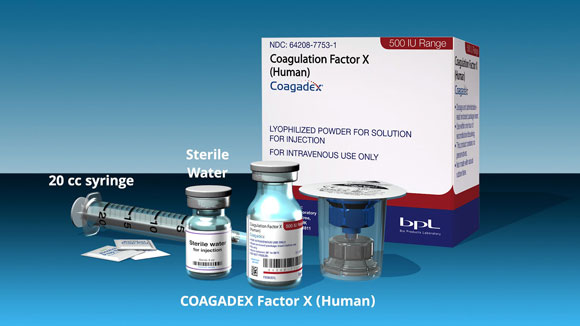
Indications and Usage for COAGADEX
COAGADEX, a plasma-derived blood coagulation factor X concentrate, is indicated in adults and children with hereditary factor X deficiency for:
- Routine prophylaxis to reduce the frequency of bleeding episodes
- On-demand treatment and control of bleeding episodes
- Perioperative management of bleeding in patients with mild, moderate and severe hereditary factor X deficiency
Contraindication for COAGADEX
COAGADEX is contraindicated in patients who have had life-threatening hypersensitivity reactions to COAGADEX.
Important Safety Information for COAGADEX
Allergic type hypersensitivity reactions, including anaphylaxis, are possible with COAGADEX. If symptoms occur, patients should discontinue use of the product immediately, contact their physician, and administer appropriate treatment.
The formation of neutralizing antibodies (inhibitors) to factor X is a possible complication in the management of individuals with factor X deficiency. Carefully monitor patients taking COAGADEX for the development of inhibitors by appropriate clinical observations and laboratory tests.
COAGADEX is made from human plasma and may contain infectious agents, e.g. viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent. No cases of transmission of viral diseases, vCJD or CJD, have been associated with the use of COAGADEX.
In clinical studies, the most common adverse reactions (frequency ≥5% of subjects) with COAGADEX were infusion site erythema, infusion site pain, fatigue and back pain.
Please see complete Prescribing Information for COAGADEX.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit https://www.fda.gov/medwatch, or call 1-800-FDA-1088.
You may also call Kedrion at 1-866-398-0825 or email US_Medicalinfo@kedrion.com.