Patient Support with COAGADEX
If you or someone you know has been recently diagnosed with HFXD and/or prescribed COAGADEX, resources are available!
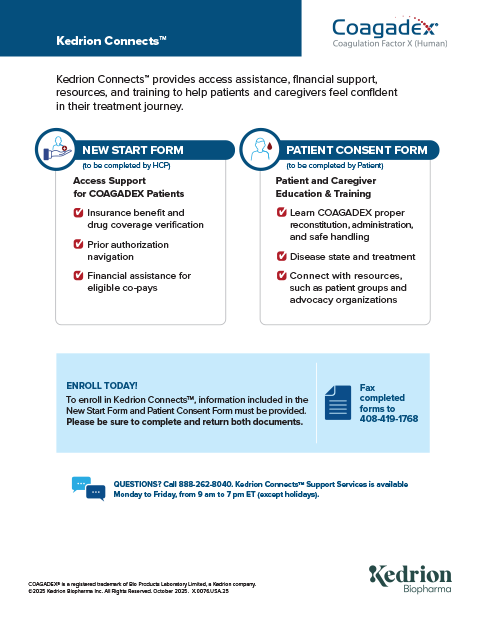
Kedrion Connects™
Patient and Caregiver Education & Training
Our Nurse Educators provide training and educational resources to help you:
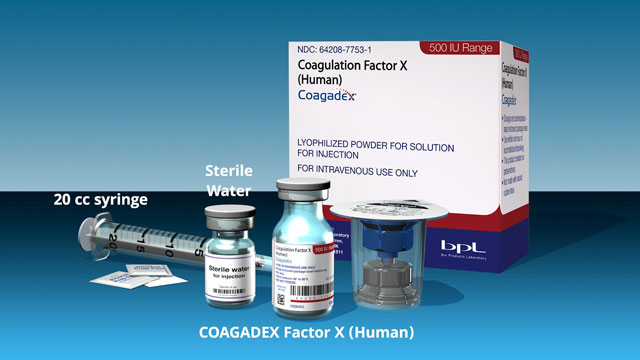
- Learn COAGADEX proper reconstitution, administration, and safe handling
- Better understand hereditary factor X deficiency and its treatment
- Connect with resources, such as patient groups and advocacy organizations
New Patient Support
We can help you navigate your treatment journey by providing assistance with:
- Insurance benefit and drug coverage verification
- Prior authorization
- Financial assistance for eligible copays
- Have your doctor or pharmacy complete the Kedrion Connects Enrollment Form to get started
Download the Kedrion Connects Enrollment Form Today
To enroll in Kedrion Connects, your healthcare provider must complete the New Start Form and Consent Form.
| Have your doctor return completed forms to MedMonk: | |
|---|---|
| Phone: | 888-262-8040 |
| Fax: | 408-419-1768 |
Copay Support
If you have a valid prescription for COAGADEX, and are having trouble paying for your medication, we may be able to help!
The COAGADEX Copay Support Program:
- Can help cover out-of-pocket expenses for a COAGADEX prescription. Some restrictions apply
- Is easy to use, with no cards required
Have your doctor or pharmacy complete the Kedrion Connects Enrollment Form to start your enrollment
Resources
Hemophilia treatment centers
Many patients with bleeding disorders seek diagnosis and treatment at a Hemophilia Treatment Center (HTC). More than 140 HTCs are found across the US. They provide high-quality care for people with all types of bleeding disorders. Talk with your doctor about HTCs.
Links for more information
Visit these sites for more information about factor X deficiency, including local and national events in the bleeding disorder community.
Specialty Pharmacies
Kedrion works with the following pharmacies to make COAGADEX available for you.
Accredo Health Group, Inc.
CVS Specialty Pharmacy
Option Care
Optum Infusion Pharmacy
Paragon Specialty
1-888-588-1072
PromptCare
Soleo Health
Connect with Us
As the maker of COAGADEX, Kedrion is here to support you.
Complete the form below to connect with us for more information or to ask a question.
You can also call toll-free: (844) 424-1010 (Monday through Friday, 9 am to 7 pm Eastern Time)


Indications and Usage for COAGADEX
COAGADEX, a plasma-derived blood coagulation factor X concentrate, is indicated in adults and children with hereditary factor X deficiency for:
- Routine prophylaxis to reduce the frequency of bleeding episodes
- On-demand treatment and control of bleeding episodes
- Perioperative management of bleeding in patients with mild, moderate and severe hereditary factor X deficiency
Contraindication for COAGADEX
COAGADEX is contraindicated in patients who have had life-threatening hypersensitivity reactions to COAGADEX.
Important Safety Information for COAGADEX
Allergic type hypersensitivity reactions, including anaphylaxis, are possible with COAGADEX. If symptoms occur, patients should discontinue use of the product immediately, contact their physician, and administer appropriate treatment.
The formation of neutralizing antibodies (inhibitors) to factor X is a possible complication in the management of individuals with factor X deficiency. Carefully monitor patients taking COAGADEX for the development of inhibitors by appropriate clinical observations and laboratory tests.
COAGADEX is made from human plasma and may contain infectious agents, e.g. viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent. No cases of transmission of viral diseases, vCJD or CJD, have been associated with the use of COAGADEX.
In clinical studies, the most common adverse reactions (frequency ≥5% of subjects) with COAGADEX were infusion site erythema, infusion site pain, fatigue and back pain.
Please see complete Prescribing Information for COAGADEX.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit https://www.fda.gov/medwatch, or call 1-800-FDA-1088.
You may also call Kedrion at 1-866-398-0825 or email US_Medicalinfo@kedrion.com.