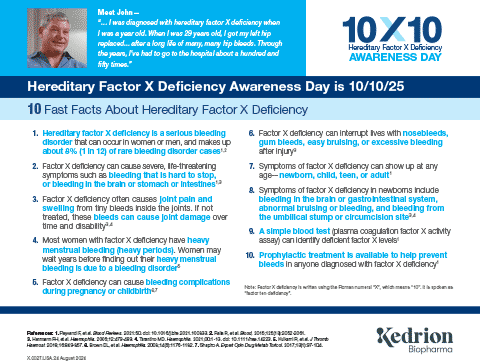
Hereditary Factor X Deficiency Is a Rare Bleeding Disorder that Affects About 1 in a Million People1,2
- Characterized by insufficient levels of factor X
- Affects both women and men
- People with factor X deficiency have a high risk of excess bleeding
- Often hereditary, as an autosomal recessive disorder

Diagnostic Indicators of Factor X Deficiency Include Bleeding Symptoms and Prolonged PT and aPTT1,2

Factor X Deficiency May Present with Ongoing Symptoms

Symptom occurrence with factor X deficiency (N=42)3*

55%

36%

33%

hemorrhage
21%
Important Note
- In this analysis, additional symptoms of factor X deficiency included hematomas (43%), gum bleeding (31%), gastrointestinal hemorrhage (12%), and hematuria (7%)
*Refers to data collected from one series of patients from three Central European and two Latin American countries in a 2006 study; numbers reflect characterization of the 42 symptomatic patients out of 102 total subjects with confirmed reduced FX activity and identified F10 gene causative mutations (<50% of the sample were symptomatic).
The majority of women with factor X deficiency have a history of heavy menstrual bleeding3

In women of reproductive age with hereditary factor X deficiency:
- Up to 70% (12/17) have heavy menstrual bleeding3†
- Risks with pregnancy include miscarriage, uterine bleeding, postpartum hemorrhage, and preterm labor
Undiagnosed and untreated neonates can be at high risk for severe bleeds3,4
Affected neonates are at risk of experiencing devastating outcomes if hereditary factor X deficiency is not promptly recognized and treated
Symptoms in neonates3,4:
- Intracranial hemorrhage (ICH)
- Gastrointestinal (GI) bleeding
- Bleeding that won’t stop normally from the umbilical stump or post-circumcision
- Abnormal bruising or bleeding
With Hereditary Factor X Deficiency, ICH and GI Bleeds are Likely to Occur Early in Life3,4
- In a study of 102 adult and pediatric patients with hereditary factor X deficiency, 42 of whom were symptomatic—
- In neonates, ICH and GI bleeds presented at a median age of 9.7 days
Use of plasma-derived factor X concentrate in neonates and infants with congenital factor X deficiency.
Severity Classification is Unique to Factor X4,6–8
SEVERE
High risk of major spontaneous bleeding
MODERATE
Minor spontaneous or triggered bleeding risk
MILD
Low bleeding risk
- Decreasing factor X activity has been shown to correlate with increasing bleeding severity7
- Severity classification as shown is currently widely accepted but differs from that existing at the time of COAGADEX clinical trials, which classified “severe” factor X deficiency activity as <1 IU/dL8
- The COAGADEX prescribing information recommendation is to monitor trough blood levels of factor X targeting ≥5 IU/dL and adjust dosage to clinical response and trough levels9
- Analysis of registry data from the European Network of Rare Bleeding Disorders (EN-RBD) suggests maintaining a factor X level of ≥10 IU/dL as the appropriate target for prophylaxis, due to the high risk of major spontaneous bleeding (such as GI or CNS) at <10 IU/dL7,10
- In the general population, factor X activity ranges from 65–120 IU/dL (reference ranges may vary)8
A review of data by the Rare Bleeding Disorders Working Group under the Factor VIII and Factor IX Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis, exploring the association between residual plasma coagulant factor activity and clinical bleeding profile for each rare bleeding disorder (RBD; N=4,359 patients with RBDs in North American, European, UK, and Indian registries). Factor X severity classification based on analysis of registry data from the European Network of RBDs (EN-RBD; n=592 patients with RBDs).
Treatment Considerations
Clinical guidelines and expert consensus recommend use of plasma-derived factor X concentrate for the treatment of hereditary factor X deficiency4,7,9

Recommendations concerning products licensed for the treatment of hemophilia and other bleeding disorders, 2022.

Diagnosis, therapeutic advances, and key recommendations for the management of factor X deficiency.
Blood Reviews. 2021.

Occurrence and management of severe bleeding episodes in patients with hereditary factor X deficiency.
Haemophilia. 2021.

Treatment of rare factor deficiencies other than hemophilia.
Blood. 2019.

References: 1. Brown DL, Kouides PA. Haemophilia. 2008;14(6):1176-1182. 2. Palla R, et al. Blood. 2015;125(13):2052-2061. 3. Hermann FH, et al. Haemophilia. 2006;12:479-489. 4. Tarantino MD. Haemophilia. 2021;27:531-543. 5. Zimowski KL, McGuinn YL, Abajas YL, et al. J Thromb Haemost. 2020;18:2551-2556. 6. Peyvandi F, Palla R, et al. J Thromb Haemost. 2012;10:615-621. 7. Peyvandi F, Auerswald G, Austin SK, et al. Blood Reviews. 2021;50:100833. 8. Peyvandi F, et al. Brit J Haematol. 1998;102:626-628. 9. COAGADEX® (Coagulation Factor X, Human) Prescribing Information. Durham, NC: BPL Limited. 2023. 10. Peyvandi F, et al. J Thromb Haemost. 2012;10:1938-1943. 11. Medical and Scientific Advisory Council (MASAC) of the National Hemophilia Foundation. MASAC Document #272. https://www.hemophilia.org/healthcare-professionals/guidelines-on-care/masac-documents/masac-document-272-masac-recommendations-concerning-products-licensed-for-the-treatment-of-hemophilia-and-other-bleeding-disorders. Accessed November 9, 2022. 12. Menegatti M, Peyvandi F. Blood. 2019;133:415-424.


Indications and Usage for COAGADEX
COAGADEX, a plasma-derived blood coagulation factor X concentrate, is indicated in adults and children with hereditary factor X deficiency for:
- Routine prophylaxis to reduce the frequency of bleeding episodes
- On-demand treatment and control of bleeding episodes
- Perioperative management of bleeding in patients with mild, moderate and severe hereditary factor X deficiency
Contraindication for COAGADEX
COAGADEX is contraindicated in patients who have had life-threatening hypersensitivity reactions to COAGADEX.
Important Safety Information for COAGADEX
Allergic type hypersensitivity reactions, including anaphylaxis, are possible with COAGADEX. If symptoms occur, patients should discontinue use of the product immediately, contact their physician, and administer appropriate treatment.
The formation of neutralizing antibodies (inhibitors) to factor X is a possible complication in the management of individuals with factor X deficiency. Carefully monitor patients taking COAGADEX for the development of inhibitors by appropriate clinical observations and laboratory tests.
COAGADEX is made from human plasma and may contain infectious agents, e.g. viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent. No cases of transmission of viral diseases, vCJD or CJD, have been associated with the use of COAGADEX.
In clinical studies, the most common adverse reactions (frequency ≥5% of subjects) with COAGADEX were infusion site erythema, infusion site pain, fatigue and back pain.
Please see complete Prescribing Information for COAGADEX.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit https://www.fda.gov/medwatch, or call 1-800-FDA-1088.
You may also call Kedrion at 1-866-398-0825 or email US_Medicalinfo@kedrion.com.